Small Ribozymes
Small ribozymes are catalytically active RNA molecules, typically ranging from 50 to 150 nucleotides in length. They mediate site-specific intramolecular cleavage reactions, producing fragments with 2′,3′-cyclic phosphate and 5′-hydroxyl ends. The main classes of small ribozymes include hammerhead, hairpin, hepatitis delta virus (HDV), Varkud Satellite (VS), and glmS ribozymes.
One of the small ribozymes of particular interest in our group is the cytoplasmic polyadenylation element-binding 3 (CPEB3) ribozyme. It is one of the very few small ribozymes found in mammalian genomes, including humans. The CPEB3 ribozyme belongs to the HDV-like family and adopts an intricate ‘nested double pseudoknot’ structure, enabling self-cleavage through a transesterification reaction. Our goal is to determine its three-dimensional structure using NMR spectroscopy and X-ray crystallography and to investigate the role of Mg²⁺ ions in its folding and catalytic mechanism.
Theta Ribozymes
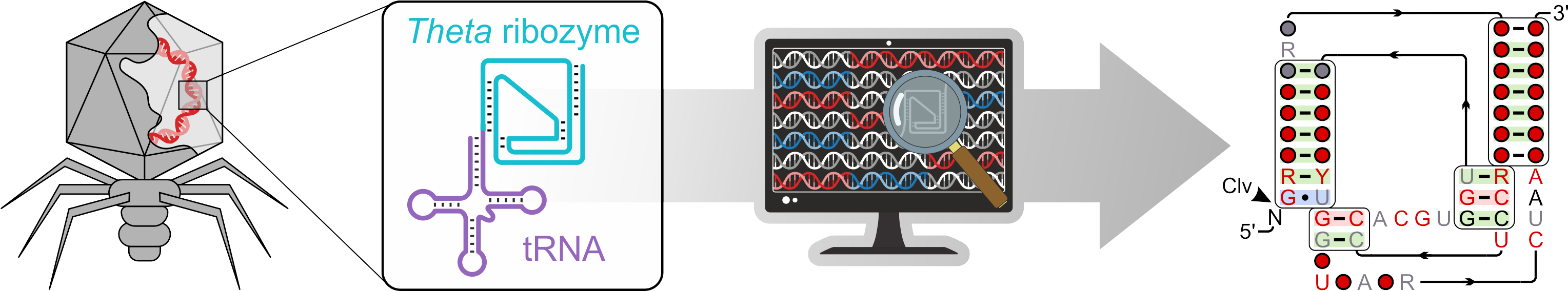
Recently, we identified minimal versions of HDV-like ribozymes associated with tRNAs, which we have termed theta ribozymes. These ribozymes appear to be restricted to bacteriophages within the mammalian gut microbiome, and our current hypothesis is that they contribute to viral tRNA-mediated host manipulation. We aim to understand the catalytic properties of theta ribozymes, which span at least two orders of magnitude in efficiency, at a molecular level. To achieve this, we employ a multidisciplinary approach that integrates bioinformatics, chemical biology, and, more recently, single-particle cryogenic electron microscopy (cryo-EM).
Literature